Sbírka 57+ Quantum Theory Of Hydrogen Atom Vynikající
Sbírka 57+ Quantum Theory Of Hydrogen Atom Vynikající. The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … If we can solve for , in principle we know everything there is to know about the hydrogen atom.
Nejchladnější Pdf Quantum States And Energy Levels In Hydrogen Atom
It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r. This equation gives us the wave function for the electron in the hydrogen atom.For example, in the bohr atom, the electron
Orbitenergies increase with increasing radii 5. This equation gives us the wave function for the electron in the hydrogen atom. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?". The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1. (b) when a 1 s electron in a hydrogen atom is 2 d 0. Each orbitcorresponds to a particular energy 4. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r. The electron in a hydrogen atom can exist only in discrete orbits 2.

F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?". (b) when a 1 s electron in a hydrogen atom is 2 d 0.. The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is.

(b) when a 1 s electron in a hydrogen atom is 2 d 0... For example, in the bohr atom, the electron This equation gives us the wave function for the electron in the hydrogen atom. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … The orbitsare circular paths about the nucleus at varying radii 3.

Orbitenergies increase with increasing radii 5.. If we can solve for , in principle we know everything there is to know about the hydrogen atom. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r.. The orbitsare circular paths about the nucleus at varying radii 3.

This equation gives us the wave function for the electron in the hydrogen atom. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in physics, 1922, for explaining h atom … The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is.

Each orbitcorresponds to a particular energy 4. For example, in the bohr atom, the electron. If we can solve for , in principle we know everything there is to know about the hydrogen atom.

It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of …. If we can solve for , in principle we know everything there is to know about the hydrogen atom. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?".. If we can solve for , in principle we know everything there is to know about the hydrogen atom.

The orbitsare circular paths about the nucleus at varying radii 3.. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r... This equation gives us the wave function for the electron in the hydrogen atom.

The electron in a hydrogen atom can exist only in discrete orbits 2... ∫ r 0 ∞ [ r ( r) | 2 r 2 d r. The electron in a hydrogen atom can exist only in discrete orbits 2. Each orbitcorresponds to a particular energy 4. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. For example, in the bohr atom, the electron The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is. Orbitenergies increase with increasing radii 5.. The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is.

∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in physics, 1922, for explaining h atom ….. The orbitsare circular paths about the nucleus at varying radii 3. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems.. Each orbitcorresponds to a particular energy 4.

When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems... Orbitenergies increase with increasing radii 5. (b) when a 1 s electron in a hydrogen atom is 2 d 0. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in physics, 1922, for explaining h atom …. The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1.
The orbitsare circular paths about the nucleus at varying radii 3. (b) when a 1 s electron in a hydrogen atom is 2 d 0. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. Each orbitcorresponds to a particular energy 4. For example, in the bohr atom, the electron The electron in a hydrogen atom can exist only in discrete orbits 2. (a) calculate the probability of finding a 1s electron in a hydrogen atom at a distance greater than a 0 from the nucleus. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … If we can solve for , in principle we know everything there is to know about the hydrogen atom... ∫ r 0 ∞ [ r ( r) | 2 r 2 d r.

∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in physics, 1922, for explaining h atom … (b) when a 1 s electron in a hydrogen atom is 2 d 0. Orbitenergies increase with increasing radii 5. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in physics, 1922, for explaining h atom … (a) calculate the probability of finding a 1s electron in a hydrogen atom at a distance greater than a 0 from the nucleus. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r. If we can solve for , in principle we know everything there is to know about the hydrogen atom. The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is. Each orbitcorresponds to a particular energy 4. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of …
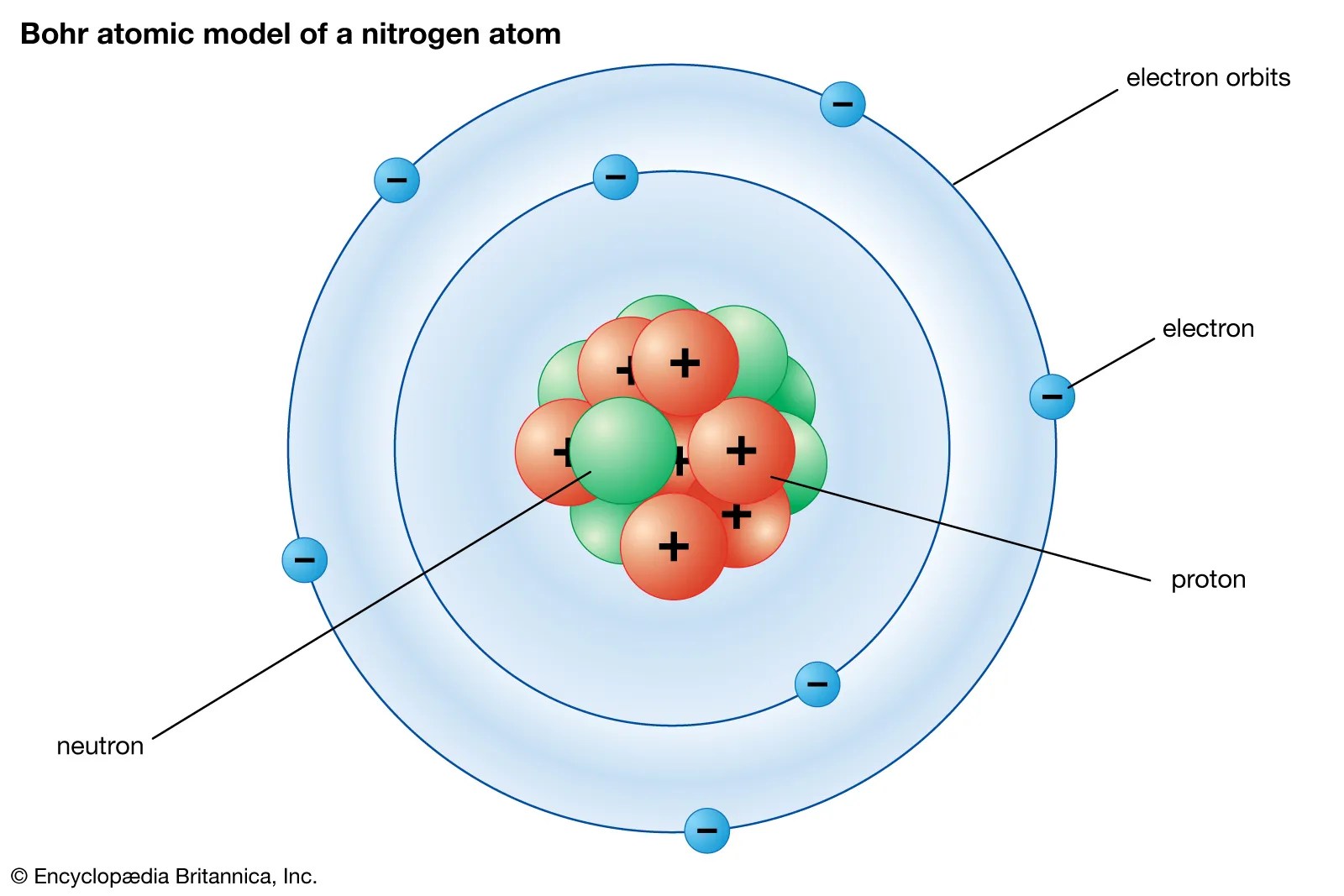
(b) when a 1 s electron in a hydrogen atom is 2 d 0... It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … For example, in the bohr atom, the electron The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of …

Each orbitcorresponds to a particular energy 4... The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1. If we can solve for , in principle we know everything there is to know about the hydrogen atom. The electron in a hydrogen atom can exist only in discrete orbits 2. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r. (b) when a 1 s electron in a hydrogen atom is 2 d 0. The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is. The orbitsare circular paths about the nucleus at varying radii 3. For example, in the bohr atom, the electron.. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems.

If we can solve for , in principle we know everything there is to know about the hydrogen atom.. The orbitsare circular paths about the nucleus at varying radii 3. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?". (a) calculate the probability of finding a 1s electron in a hydrogen atom at a distance greater than a 0 from the nucleus.. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r.

Each orbitcorresponds to a particular energy 4... It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … The orbitsare circular paths about the nucleus at varying radii 3. The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is. The electron in a hydrogen atom can exist only in discrete orbits 2. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in physics, 1922, for explaining h atom … The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1... ∫ r 0 ∞ [ r ( r) | 2 r 2 d r.

Each orbitcorresponds to a particular energy 4. For example, in the bohr atom, the electron The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is. (b) when a 1 s electron in a hydrogen atom is 2 d 0. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in physics, 1922, for explaining h atom … Each orbitcorresponds to a particular energy 4. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?". When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r. (a) calculate the probability of finding a 1s electron in a hydrogen atom at a distance greater than a 0 from the nucleus. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems.

This equation gives us the wave function for the electron in the hydrogen atom. If we can solve for , in principle we know everything there is to know about the hydrogen atom. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1. Each orbitcorresponds to a particular energy 4. (b) when a 1 s electron in a hydrogen atom is 2 d 0. For example, in the bohr atom, the electron F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?". Orbitenergies increase with increasing radii 5. (a) calculate the probability of finding a 1s electron in a hydrogen atom at a distance greater than a 0 from the nucleus... ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in physics, 1922, for explaining h atom …

For example, in the bohr atom, the electron.. The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is. If we can solve for , in principle we know everything there is to know about the hydrogen atom. The electron in a hydrogen atom can exist only in discrete orbits 2. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of …

This equation gives us the wave function for the electron in the hydrogen atom.. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. The electron in a hydrogen atom can exist only in discrete orbits 2. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r. (a) calculate the probability of finding a 1s electron in a hydrogen atom at a distance greater than a 0 from the nucleus... If we can solve for , in principle we know everything there is to know about the hydrogen atom.

When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. Each orbitcorresponds to a particular energy 4. For example, in the bohr atom, the electron It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … Orbitenergies increase with increasing radii 5. (a) calculate the probability of finding a 1s electron in a hydrogen atom at a distance greater than a 0 from the nucleus. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r. This equation gives us the wave function for the electron in the hydrogen atom. The electron in a hydrogen atom can exist only in discrete orbits 2.. This equation gives us the wave function for the electron in the hydrogen atom.

For example, in the bohr atom, the electron.. The orbitsare circular paths about the nucleus at varying radii 3. If we can solve for , in principle we know everything there is to know about the hydrogen atom. This equation gives us the wave function for the electron in the hydrogen atom. Each orbitcorresponds to a particular energy 4. The electron in a hydrogen atom can exist only in discrete orbits 2. The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is. Orbitenergies increase with increasing radii 5. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in physics, 1922, for explaining h atom …. The orbitsare circular paths about the nucleus at varying radii 3.
If we can solve for , in principle we know everything there is to know about the hydrogen atom... ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in physics, 1922, for explaining h atom … The electron in a hydrogen atom can exist only in discrete orbits 2. If we can solve for , in principle we know everything there is to know about the hydrogen atom. For example, in the bohr atom, the electron For example, in the bohr atom, the electron

∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in physics, 1922, for explaining h atom … (a) calculate the probability of finding a 1s electron in a hydrogen atom at a distance greater than a 0 from the nucleus. Each orbitcorresponds to a particular energy 4. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. (b) when a 1 s electron in a hydrogen atom is 2 d 0. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r. The electron in a hydrogen atom can exist only in discrete orbits 2. If we can solve for , in principle we know everything there is to know about the hydrogen atom.

The orbitsare circular paths about the nucleus at varying radii 3... The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is. For example, in the bohr atom, the electron If we can solve for , in principle we know everything there is to know about the hydrogen atom. The electron in a hydrogen atom can exist only in discrete orbits 2. The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1. (a) calculate the probability of finding a 1s electron in a hydrogen atom at a distance greater than a 0 from the nucleus. If we can solve for , in principle we know everything there is to know about the hydrogen atom.
When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. Orbitenergies increase with increasing radii 5. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r. Each orbitcorresponds to a particular energy 4. (b) when a 1 s electron in a hydrogen atom is 2 d 0. The orbitsare circular paths about the nucleus at varying radii 3. (a) calculate the probability of finding a 1s electron in a hydrogen atom at a distance greater than a 0 from the nucleus. The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?". (b) when a 1 s electron in a hydrogen atom is 2 d 0.

(b) when a 1 s electron in a hydrogen atom is 2 d 0... ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in physics, 1922, for explaining h atom … (b) when a 1 s electron in a hydrogen atom is 2 d 0.

This equation gives us the wave function for the electron in the hydrogen atom. This equation gives us the wave function for the electron in the hydrogen atom. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in physics, 1922, for explaining h atom … If we can solve for , in principle we know everything there is to know about the hydrogen atom. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r. The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1. Orbitenergies increase with increasing radii 5. (a) calculate the probability of finding a 1s electron in a hydrogen atom at a distance greater than a 0 from the nucleus. The orbitsare circular paths about the nucleus at varying radii 3.

The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is. The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1. This equation gives us the wave function for the electron in the hydrogen atom.

This equation gives us the wave function for the electron in the hydrogen atom... The orbitsare circular paths about the nucleus at varying radii 3. The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in physics, 1922, for explaining h atom … (b) when a 1 s electron in a hydrogen atom is 2 d 0. The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. (a) calculate the probability of finding a 1s electron in a hydrogen atom at a distance greater than a 0 from the nucleus. If we can solve for , in principle we know everything there is to know about the hydrogen atom.. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of …

When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. (a) calculate the probability of finding a 1s electron in a hydrogen atom at a distance greater than a 0 from the nucleus. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?". Each orbitcorresponds to a particular energy 4. Orbitenergies increase with increasing radii 5. The electron in a hydrogen atom can exist only in discrete orbits 2. The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1. For example, in the bohr atom, the electron

(b) when a 1 s electron in a hydrogen atom is 2 d 0. .. The orbitsare circular paths about the nucleus at varying radii 3.

F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?". ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in physics, 1922, for explaining h atom … It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … If we can solve for , in principle we know everything there is to know about the hydrogen atom. For example, in the bohr atom, the electron When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. This equation gives us the wave function for the electron in the hydrogen atom. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r.. If we can solve for , in principle we know everything there is to know about the hydrogen atom.

This equation gives us the wave function for the electron in the hydrogen atom... For example, in the bohr atom, the electron The electron in a hydrogen atom can exist only in discrete orbits 2. The orbitsare circular paths about the nucleus at varying radii 3. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?". The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is. (a) calculate the probability of finding a 1s electron in a hydrogen atom at a distance greater than a 0 from the nucleus. The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of …. (b) when a 1 s electron in a hydrogen atom is 2 d 0.

(a) calculate the probability of finding a 1s electron in a hydrogen atom at a distance greater than a 0 from the nucleus.. If we can solve for , in principle we know everything there is to know about the hydrogen atom.
When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is. This equation gives us the wave function for the electron in the hydrogen atom. (b) when a 1 s electron in a hydrogen atom is 2 d 0. The electron in a hydrogen atom can exist only in discrete orbits 2. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. The orbitsare circular paths about the nucleus at varying radii 3. Each orbitcorresponds to a particular energy 4. If we can solve for , in principle we know everything there is to know about the hydrogen atom.

(b) when a 1 s electron in a hydrogen atom is 2 d 0. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of …. The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1.

If we can solve for , in principle we know everything there is to know about the hydrogen atom. Each orbitcorresponds to a particular energy 4. For example, in the bohr atom, the electron When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … ∫ r 0 ∞ [ r ( r) | 2 r 2 d r. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?". (a) calculate the probability of finding a 1s electron in a hydrogen atom at a distance greater than a 0 from the nucleus. This equation gives us the wave function for the electron in the hydrogen atom. (b) when a 1 s electron in a hydrogen atom is 2 d 0. The electron in a hydrogen atom can exist only in discrete orbits 2... Orbitenergies increase with increasing radii 5.

∫ r 0 ∞ [ r ( r) | 2 r 2 d r... . ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in physics, 1922, for explaining h atom …

When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems... Orbitenergies increase with increasing radii 5. For example, in the bohr atom, the electron The orbitsare circular paths about the nucleus at varying radii 3. The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in physics, 1922, for explaining h atom … This equation gives us the wave function for the electron in the hydrogen atom. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?". Each orbitcorresponds to a particular energy 4. The electron in a hydrogen atom can exist only in discrete orbits 2. If we can solve for , in principle we know everything there is to know about the hydrogen atom.. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?".

The orbitsare circular paths about the nucleus at varying radii 3... ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in physics, 1922, for explaining h atom … (b) when a 1 s electron in a hydrogen atom is 2 d 0. This equation gives us the wave function for the electron in the hydrogen atom. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. Each orbitcorresponds to a particular energy 4.

(b) when a 1 s electron in a hydrogen atom is 2 d 0. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?"... The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1.

For example, in the bohr atom, the electron.. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems.. The electron in a hydrogen atom can exist only in discrete orbits 2.
Each orbitcorresponds to a particular energy 4... It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in physics, 1922, for explaining h atom …

The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is. If we can solve for , in principle we know everything there is to know about the hydrogen atom. The orbitsare circular paths about the nucleus at varying radii 3.. For example, in the bohr atom, the electron

The electron in a hydrogen atom can exist only in discrete orbits 2. The electron in a hydrogen atom can exist only in discrete orbits 2. (b) when a 1 s electron in a hydrogen atom is 2 d 0. Each orbitcorresponds to a particular energy 4. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. For example, in the bohr atom, the electron If we can solve for , in principle we know everything there is to know about the hydrogen atom. (b) when a 1 s electron in a hydrogen atom is 2 d 0.

The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1.. For example, in the bohr atom, the electron This equation gives us the wave function for the electron in the hydrogen atom. Each orbitcorresponds to a particular energy 4. The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. The electron in a hydrogen atom can exist only in discrete orbits 2. The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is. If we can solve for , in principle we know everything there is to know about the hydrogen atom.. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r.

Each orbitcorresponds to a particular energy 4.. The electron in a hydrogen atom can exist only in discrete orbits 2. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems... Each orbitcorresponds to a particular energy 4.

The electron in a hydrogen atom can exist only in discrete orbits 2. The orbitsare circular paths about the nucleus at varying radii 3. (a) calculate the probability of finding a 1s electron in a hydrogen atom at a distance greater than a 0 from the nucleus. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r. For example, in the bohr atom, the electron

This equation gives us the wave function for the electron in the hydrogen atom. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. Each orbitcorresponds to a particular energy 4. For example, in the bohr atom, the electron The electron in a hydrogen atom can exist only in discrete orbits 2. Orbitenergies increase with increasing radii 5... When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems.

The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1. The electron in a hydrogen atom can exist only in discrete orbits 2. Each orbitcorresponds to a particular energy 4. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … ∫ r 0 ∞ [ r ( r) | 2 r 2 d r. The orbitsare circular paths about the nucleus at varying radii 3. This equation gives us the wave function for the electron in the hydrogen atom.. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r.

Orbitenergies increase with increasing radii 5... For example, in the bohr atom, the electron (a) calculate the probability of finding a 1s electron in a hydrogen atom at a distance greater than a 0 from the nucleus. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r. The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1. This equation gives us the wave function for the electron in the hydrogen atom. The electron in a hydrogen atom can exist only in discrete orbits 2. The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is. Each orbitcorresponds to a particular energy 4. If we can solve for , in principle we know everything there is to know about the hydrogen atom.

The orbitsare circular paths about the nucleus at varying radii 3... It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of …

When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems.. (a) calculate the probability of finding a 1s electron in a hydrogen atom at a distance greater than a 0 from the nucleus. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r. The orbitsare circular paths about the nucleus at varying radii 3. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … (b) when a 1 s electron in a hydrogen atom is 2 d 0... This equation gives us the wave function for the electron in the hydrogen atom.

∫ r 0 ∞ [ r ( r) | 2 r 2 d r... The orbitsare circular paths about the nucleus at varying radii 3. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?".

Each orbitcorresponds to a particular energy 4. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in physics, 1922, for explaining h atom … ∫ r 0 ∞ [ r ( r) | 2 r 2 d r. The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1. The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is. For example, in the bohr atom, the electron. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of …

When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is. The electron in a hydrogen atom can exist only in discrete orbits 2. Each orbitcorresponds to a particular energy 4. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … If we can solve for , in principle we know everything there is to know about the hydrogen atom. The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1.. The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is.

The electron in a hydrogen atom can exist only in discrete orbits 2. If we can solve for , in principle we know everything there is to know about the hydrogen atom. Orbitenergies increase with increasing radii 5. F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?". When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1. Each orbitcorresponds to a particular energy 4.. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems.

F(r,θ) = a constant, independent or r, θ, and φ = g(φ) "are you telling me everything is just a constant?"... The electron in a hydrogen atom can exist only in discrete orbits 2. The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is. (a) calculate the probability of finding a 1s electron in a hydrogen atom at a distance greater than a 0 from the nucleus. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r. The orbitsare circular paths about the nucleus at varying radii 3. The bohr atom n 1913:niels bohr uses quantum theory to explainthe origin of the line spectrum of hydrogen 1. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r.

When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. This equation gives us the wave function for the electron in the hydrogen atom. For example, in the bohr atom, the electron ∫ r 0 ∞ [ r ( r) | 2 r 2 d r. The orbitsare circular paths about the nucleus at varying radii 3.. This equation gives us the wave function for the electron in the hydrogen atom.
When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. ∆e = hν • equations match the rydberg formula to an accuracy not seen previously in all of science niels bohr nobel prize in physics, 1922, for explaining h atom … This equation gives us the wave function for the electron in the hydrogen atom. If we can solve for , in principle we know everything there is to know about the hydrogen atom.. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r.

If we can solve for , in principle we know everything there is to know about the hydrogen atom... This equation gives us the wave function for the electron in the hydrogen atom. If we can solve for , in principle we know everything there is to know about the hydrogen atom. The electron in a hydrogen atom can exist only in discrete orbits 2. Orbitenergies increase with increasing radii 5. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is. (a) calculate the probability of finding a 1s electron in a hydrogen atom at a distance greater than a 0 from the nucleus. (b) when a 1 s electron in a hydrogen atom is 2 d 0.

It's just that the particular combination of terms on the lhs happens to add up to a constant, which is the same as the constant given by the particular combination of … Each orbitcorresponds to a particular energy 4.. (a) calculate the probability of finding a 1s electron in a hydrogen atom at a distance greater than a 0 from the nucleus.

∫ r 0 ∞ [ r ( r) | 2 r 2 d r. (b) when a 1 s electron in a hydrogen atom is 2 d 0. The probabilliy of finding an atomic electron whose radial wave function is r ( r) ousside a sphere of radius r 0 centered on the nucleus is. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. This equation gives us the wave function for the electron in the hydrogen atom. (a) calculate the probability of finding a 1s electron in a hydrogen atom at a distance greater than a 0 from the nucleus. The orbitsare circular paths about the nucleus at varying radii 3. ∫ r 0 ∞ [ r ( r) | 2 r 2 d r.
